Which Describes Ernest Rutherford's Experiment
The plum pudding model of J. The positively charged particles were deflected because like charges repel.

Rutherford S Atomic Model Chemistry For Non Majors
2 on a question.

. Which answer choice best describes why the results of Ernest Rutherfords gold foil experiment led him to propose the concept of a nucleus. What did Ernest Rutherfords gold foil experiment demonstrate about atoms. Rutherfords Alpha Scattering Experiment.
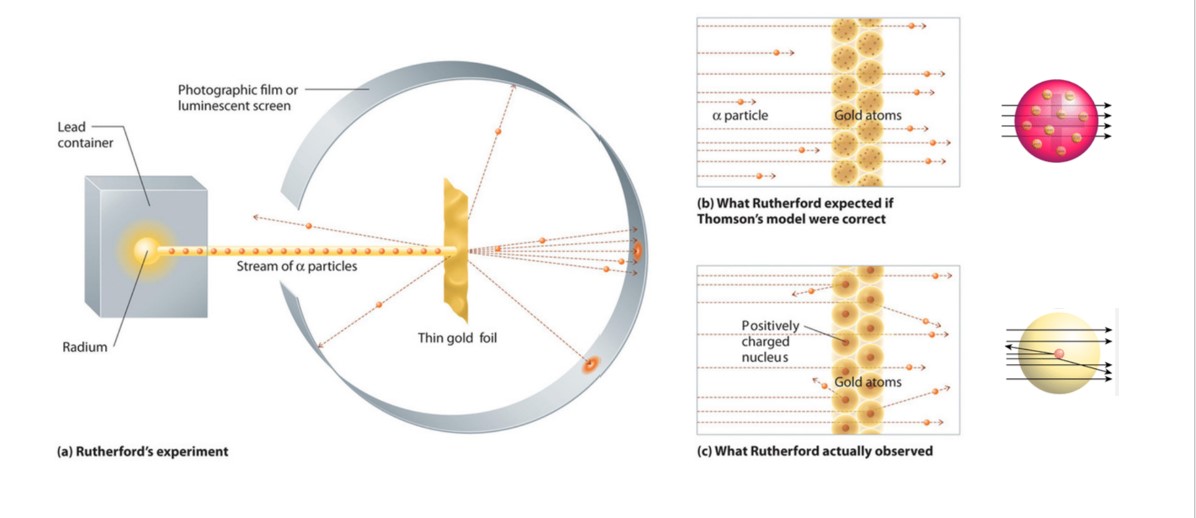
The best description of Ernest Rutherfords experiment is letter C. Most bounce back but some pass through. This is the correct answer because.
Bnegative particles are fired. Which statements accurately describe Ernest Rutherfords experiment. In Ernest Rutherfords experiment he used a beam of alpha particles which were aimed at very thin gold foil and their passage.
Most bounce back but some pass through. Most pass through but some bounce back. Which describes ernest rutherfords experiment.
The positively charged alpha particles were. Check all that apply. He realized this because most of the alpha.
Rutherfords gold foil experiment showed that atoms are mostly empty space with the positive charge concentrated in a nucleus. Apositive particles are fired at gold foil. Apositive particles are fired at gold foil.
The energy needed to split an atom into separate protons neutrons and electrons. Most pass through but some bounce back best describes Ernest Rutherfords experiment. Which describes ernest rutherfords experiment.
Their positive charge is located in a small region that is called the nucleus 2Their negative charge is. Thomson could not able to explain certain experimental results about. Which describes ernest rutherfords experiment.
Bnegative particles are fired at gold foil. The statement Positive particles are fired at a gold foil. Apositive particles are fired at gold foil.
The positively charged particles were fired through a gold foil. Which quantity can be calculated using the equation E mc2. Most bounce back but some pass through.

Rutherford S Atomic Model Physics8atlaurel
Experimental Evidence For The Structure Of The Atom

The Atom Electron Configuration Ernest Rutherford Chemistry Help
3 4 Rutherford S Experiment The Nuclear Model Of The Atom Chemistry Libretexts
Comments
Post a Comment